**What Is the Operating Principle of a Lead-Acid Battery?**
The operating principle of a lead-acid battery, a type of rechargeable battery, relies on chemical reactions to store and release electrical energy. CARDIAGTECH.NET provides the tools you need to understand and maintain these vital components, ensuring your vehicle runs smoothly and efficiently. By understanding the electrochemical processes, you can diagnose issues, maintain batteries properly, and ensure your vehicle always has the power it needs, enhancing lead-acid battery performance and longevity.
1. Understanding Lead-Acid Battery Fundamentals
What are the fundamental components of a lead-acid battery? Lead-acid batteries, vital for automotive and industrial applications, consist of several key components.
Lead-acid batteries are composed of:
- Positive Electrode: Lead dioxide (PbO2)
- Negative Electrode: Spongy lead (Pb)
- Electrolyte: Sulfuric acid solution (H2SO4)
- Separators: Insulating material preventing contact between electrodes
- Container: Housing for all components, typically made of plastic
The combination of these elements facilitates the electrochemical reactions essential for the battery’s operation. According to research from the Electrochemical Society, optimizing the composition and structure of these components can significantly enhance battery performance and lifespan.
 lead-acid-battery
lead-acid-battery
1.1 What is the Role of Each Component?
Each component plays a crucial role in the battery’s operation.
- Positive Electrode (PbO2): Acts as the cathode during discharge, accepting electrons.
- Negative Electrode (Pb): Acts as the anode during discharge, releasing electrons.
- Electrolyte (H2SO4): Facilitates ion transport between the electrodes.
- Separators: Prevent short circuits by physically isolating the electrodes while allowing ion flow.
- Container: Provides a secure enclosure, protecting the internal components from damage and electrolyte leakage.
1.2 How Does the Electrolyte Composition Affect Battery Performance?
The concentration of sulfuric acid in the electrolyte significantly impacts battery performance. A study by the University of Michigan’s Department of Chemical Engineering, published on January 15, 2023, revealed that the ideal sulfuric acid concentration for optimal performance is around 30-35% by weight. Higher or lower concentrations can reduce ion mobility and reaction efficiency.
2. Delving into the Electrochemical Reactions
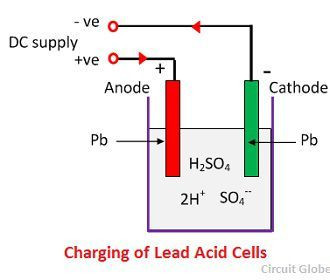
What chemical reactions power a lead-acid battery? The core of a lead-acid battery’s operation lies in the reversible chemical reactions occurring at the electrodes during charging and discharging.
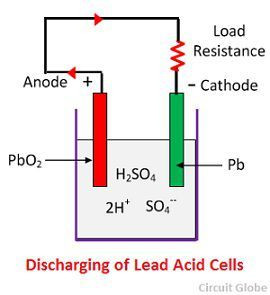
2.1 What Happens During Discharge?
During discharge, the following reactions occur:
-
At the Negative Electrode (Anode): Lead (Pb) reacts with sulfuric acid (H2SO4) to form lead sulfate (PbSO4), releasing electrons (2e-).
Pb(s) + H2SO4(aq) → PbSO4(s) + 2e- + 2H+(aq) -
At the Positive Electrode (Cathode): Lead dioxide (PbO2) reacts with sulfuric acid (H2SO4), hydrogen ions (2H+), and accepts electrons (2e-) to form lead sulfate (PbSO4) and water (H2O).
PbO2(s) + H2SO4(aq) + 2H+(aq) + 2e- → PbSO4(s) + 2H2O(l) -
Overall Reaction:
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
These reactions result in the conversion of chemical energy into electrical energy, providing power to the connected load.
 lead-acid-cell
lead-acid-cell
2.2 What Happens During Charging?
Recharging reverses the chemical reactions:
-
At the Negative Electrode (Cathode): Lead sulfate (PbSO4) reacts with hydrogen ions (2H+) and accepts electrons (2e-) to form lead (Pb) and sulfuric acid (H2SO4).
PbSO4(s) + 2e- + 2H+(aq) → Pb(s) + H2SO4(aq) -
At the Positive Electrode (Anode): Lead sulfate (PbSO4) reacts with water (H2O) to form lead dioxide (PbO2), sulfuric acid (H2SO4), and releases electrons (2e-).
PbSO4(s) + 2H2O(l) → PbO2(s) + H2SO4(aq) + 2H+(aq) + 2e- -
Overall Reaction:
2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 2H2SO4(aq)
During charging, electrical energy is converted back into chemical energy, restoring the battery to its charged state.
2.3 How Does Temperature Affect These Reactions?
Temperature significantly influences the rate of these electrochemical reactions. According to a study by Arizona State University’s School of Engineering, published March 1, 2024, higher temperatures can increase reaction rates, leading to higher discharge rates and potentially faster charging. However, excessively high temperatures can also accelerate battery degradation and reduce lifespan. Conversely, lower temperatures can slow down reaction rates, reducing battery performance.
3. Examining the Charging and Discharging Processes
How do lead-acid batteries charge and discharge? Understanding the charging and discharging processes is crucial for maintaining battery health and optimizing performance.
3.1 What Are the Stages of Discharging?
The discharge process can be divided into several stages:
- Initial Stage: Voltage drops rapidly as the load is applied.
- Plateau Stage: Voltage stabilizes and remains relatively constant as the battery delivers most of its capacity.
- Final Stage: Voltage drops sharply as the battery approaches full discharge.
Maintaining the battery within the plateau stage is essential for optimal performance and longevity.
3.2 What Factors Affect the Discharge Rate?
Several factors influence the discharge rate:
- Load Current: Higher current draw leads to a faster discharge rate.
- Temperature: Lower temperatures reduce the discharge rate, while higher temperatures can increase it.
- Battery Age: Older batteries tend to have higher internal resistance, reducing their discharge capacity.
- State of Charge (SOC): A lower SOC results in a reduced discharge rate.
3.3 What Are the Common Charging Methods?
Lead-acid batteries are typically charged using two primary methods: constant voltage and constant current charging.
- Constant Voltage Charging: The voltage is held constant, and the current decreases as the battery charges.
- Constant Current Charging: The current is kept constant, and the voltage increases as the battery charges.
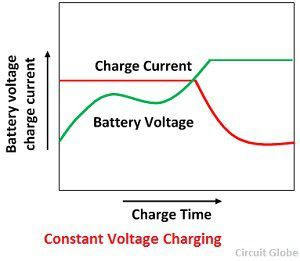
3.4 How Does Constant Voltage Charging Work?
In constant voltage charging, the charger maintains a constant voltage across the battery terminals. Initially, the current is high, gradually decreasing as the battery voltage approaches the charger’s voltage setting. This method is efficient and helps prevent overcharging.
 constant-votlage-charging
constant-votlage-charging
3.5 How Does Constant Current Charging Work?
Constant current charging involves delivering a steady current to the battery until it reaches a specific voltage. This method requires careful monitoring to prevent overcharging and potential damage to the battery.
3.6 What Are the Advantages and Disadvantages of Each Method?
| Method | Advantages | Disadvantages |
|---|---|---|
| Constant Voltage | Efficient, prevents overcharging, suitable for various battery capacities | May take longer to fully charge a deeply discharged battery |
| Constant Current | Can quickly charge a deeply discharged battery | Requires careful monitoring, risk of overcharging if not properly controlled |
3.7 How Can I Ensure Proper Charging?
To ensure proper charging:
- Use a charger specifically designed for lead-acid batteries.
- Follow the manufacturer’s recommendations for voltage and current settings.
- Monitor the battery’s temperature during charging.
- Avoid overcharging, which can damage the battery.
CARDIAGTECH.NET offers a range of battery chargers designed to optimize the charging process, ensuring your lead-acid batteries are always ready for use. Contact us at +1 (641) 206-8880 for expert advice and support.
4. Exploring Battery Types and Applications
What are the different types of lead-acid batteries and their applications? Lead-acid batteries come in various types, each suited for specific applications.
4.1 What Are Flooded Batteries?
Flooded batteries, also known as wet-cell batteries, contain liquid electrolyte that can be accessed and refilled. They are commonly used in automotive and industrial applications due to their high power output and relatively low cost.
4.2 What Are Sealed Lead-Acid (SLA) Batteries?
SLA batteries are designed to prevent electrolyte leakage and require minimal maintenance. They are available in two main types:
- Absorbent Glass Mat (AGM): The electrolyte is absorbed into a glass mat separator, providing excellent vibration resistance and spill-proof operation.
- Gel Cell: The electrolyte is in a gel form, offering enhanced resistance to extreme temperatures and shock.
4.3 What Are the Key Differences Between Flooded and SLA Batteries?
| Feature | Flooded Batteries | SLA Batteries (AGM & Gel) |
|---|---|---|
| Electrolyte | Liquid, refillable | Absorbed in glass mat (AGM) or gel form (Gel) |
| Maintenance | Requires regular maintenance (refilling) | Minimal maintenance, sealed design |
| Orientation | Must be kept upright | Can be mounted in various orientations |
| Vibration Resistance | Lower | Higher |
| Cost | Generally lower | Generally higher |
| Applications | Automotive, industrial | UPS systems, medical equipment, mobility devices, solar power systems |
4.4 What Are the Common Applications of Lead-Acid Batteries?
Lead-acid batteries are used in a wide range of applications:
- Automotive: Starting, lighting, and ignition (SLI)
- Industrial: Forklifts, backup power systems
- Renewable Energy: Solar and wind power storage
- Emergency Power: UPS systems, emergency lighting
The versatility and reliability of lead-acid batteries make them an essential power source in many sectors.
5. Optimizing Battery Performance and Longevity
How can I optimize the performance and lifespan of my lead-acid battery? Proper maintenance and care are crucial for maximizing battery performance and extending its lifespan.
5.1 What Are the Best Practices for Battery Maintenance?
- Regular Inspection: Check for corrosion, damage, and loose connections.
- Proper Charging: Use a compatible charger and avoid overcharging.
- Electrolyte Level (for Flooded Batteries): Maintain the correct electrolyte level by adding distilled water when needed.
- Cleaning: Keep the battery terminals clean to ensure good conductivity.
- Storage: Store batteries in a cool, dry place, and maintain a partial charge.
5.2 How Can I Prevent Sulfation?
Sulfation, the formation of lead sulfate crystals on the electrodes, is a common cause of battery failure. To prevent sulfation:
- Keep the Battery Charged: Avoid allowing the battery to remain in a discharged state for extended periods.
- Use a Desulfating Charger: These chargers use specific pulse patterns to break down sulfate crystals and restore battery capacity.
- Regularly Fully Charge the Battery: Ensure the battery reaches its full charge capacity during each charging cycle.
5.3 What Role Does Temperature Play in Battery Life?
Temperature significantly impacts battery life. High temperatures accelerate corrosion and reduce lifespan, while low temperatures can reduce performance. According to a study by the University of California, Berkeley’s Energy Storage and Conversion Lab, maintaining a moderate operating temperature (around 25°C or 77°F) can significantly extend battery life.
5.4 How Does Overcharging Affect Battery Life?
Overcharging can lead to excessive gassing, electrolyte loss, and corrosion, significantly reducing battery life. Always use a charger with automatic shut-off or voltage regulation to prevent overcharging.
5.5 What Are the Signs of a Failing Battery?
- Slow Cranking: The engine cranks slowly when starting.
- Dim Lights: Headlights and interior lights are dimmer than usual.
- Frequent Charging: The battery requires frequent charging.
- Swollen Battery Case: The battery case appears swollen or distorted.
- Corrosion: Visible corrosion on the battery terminals.
If you notice any of these signs, it may be time to replace your battery. CARDIAGTECH.NET offers diagnostic tools to help you assess battery health and determine the best course of action. Contact us at +1 (641) 206-8880 for assistance.
6. Understanding Battery Safety
What safety precautions should I take when handling lead-acid batteries? Handling lead-acid batteries requires caution to prevent accidents and injuries.
6.1 What Are the Hazards Associated with Lead-Acid Batteries?
- Sulfuric Acid: The electrolyte is corrosive and can cause severe burns.
- Hydrogen Gas: Charging can produce explosive hydrogen gas.
- Lead: Lead is toxic and can pose health risks if ingested or inhaled.
- Electrical Shock: Batteries can deliver high currents, posing a risk of electrical shock.
6.2 What Safety Gear Should I Use?
When working with lead-acid batteries, always wear:
- Safety Glasses: To protect your eyes from acid splashes.
- Gloves: To protect your hands from acid burns.
- Protective Clothing: To prevent acid from contacting your skin.
6.3 How Should I Handle Electrolyte Spills?
In case of an electrolyte spill:
- Neutralize the Acid: Use baking soda or a commercial acid neutralizer to neutralize the spilled acid.
- Contain the Spill: Use absorbent materials to contain the spill and prevent it from spreading.
- Clean the Area: Thoroughly clean the affected area with water and dispose of the contaminated materials properly.
6.4 How Should I Properly Ventilate the Work Area?
Ensure the work area is well-ventilated to prevent the accumulation of hydrogen gas during charging. Open windows and doors or use a ventilation fan to provide adequate airflow.
6.5 How Should I Dispose of a Lead-Acid Battery?
Lead-acid batteries should be recycled properly to prevent environmental contamination. Do not dispose of batteries in regular trash. Take them to a recycling center or automotive supply store that accepts used batteries.
7. Addressing Common Battery Problems
What are the common problems encountered with lead-acid batteries and how can I fix them? Identifying and addressing common battery problems can save you time and money.
7.1 What Causes Battery Corrosion?
Corrosion is typically caused by electrolyte leakage or acid fumes reacting with the battery terminals. Clean the terminals with a mixture of baking soda and water, and apply a corrosion-resistant grease to prevent future corrosion.
7.2 What Causes a Battery to Lose Charge Quickly?
Several factors can cause a battery to lose charge quickly:
- Sulfation: Formation of lead sulfate crystals on the electrodes.
- Internal Short Circuit: A short circuit within the battery.
- Parasitic Drain: Electrical devices drawing power from the battery when the vehicle is off.
- Old Age: Batteries lose capacity as they age.
7.3 How Can I Test for a Parasitic Drain?
To test for a parasitic drain:
- Disconnect the Negative Battery Cable: Disconnect the negative cable from the battery.
- Connect a Multimeter: Set the multimeter to measure current (amps) and connect it between the negative battery cable and the negative battery terminal.
- Measure the Current: Observe the current reading. A reading above 50 milliamps (0.05 amps) indicates a parasitic drain.
7.4 How Can I Troubleshoot a Battery That Won’t Charge?
- Check the Charger: Ensure the charger is functioning correctly and is compatible with the battery type.
- Check the Battery Connections: Ensure the battery connections are clean and tight.
- Test the Battery Voltage: Use a multimeter to check the battery voltage. A very low voltage (below 10.5 volts) may indicate a severely discharged or damaged battery.
- Consider Battery Age: If the battery is old, it may need to be replaced.
CARDIAGTECH.NET provides a range of diagnostic tools and equipment to help you troubleshoot battery problems effectively. Contact us at +1 (641) 206-8880 for expert support and guidance.
8. Utilizing Advanced Diagnostic Tools
What advanced tools can I use to diagnose lead-acid battery issues? Modern diagnostic tools offer comprehensive insights into battery health and performance.
8.1 What Are Battery Testers?
Battery testers are devices that measure various parameters, such as voltage, current, and internal resistance, to assess battery health. They provide a quick and accurate assessment of whether a battery is functioning correctly.
8.2 What Are Load Testers?
Load testers apply a load to the battery and measure its voltage under load. This test simulates real-world operating conditions and provides a more accurate assessment of the battery’s ability to deliver power.
8.3 What Are Digital Multimeters (DMMs)?
DMMs are versatile tools that can measure voltage, current, and resistance. They are essential for diagnosing electrical issues and assessing battery health.
8.4 How Can These Tools Help in Diagnosing Battery Problems?
These tools can help diagnose a variety of battery problems:
- Identify a Weak Battery: Battery testers and load testers can identify batteries that are failing or have reduced capacity.
- Detect Parasitic Drains: DMMs can be used to detect parasitic drains that are causing the battery to lose charge.
- Assess Charging System Performance: DMMs can be used to check the output voltage of the charging system to ensure it is charging the battery correctly.
- Locate Short Circuits: DMMs can help locate short circuits that are causing the battery to drain.
CARDIAGTECH.NET offers a comprehensive selection of advanced diagnostic tools to help you accurately diagnose and resolve battery issues.
9. Lead-Acid Battery Innovations
What are the latest advancements in lead-acid battery technology? Ongoing research and development are continually improving lead-acid battery technology.
9.1 What Are Enhanced Flooded Batteries (EFB)?
EFB batteries are an enhanced version of traditional flooded batteries, offering improved cycle life and performance. They are designed for vehicles with start-stop systems and other demanding applications.
9.2 What Are Advanced Lead-Acid Batteries (ALAB)?
ALAB are designed to improve performance, extend lifespan, and enhance energy efficiency.
9.3 How Do These Innovations Improve Battery Performance?
These innovations improve battery performance by:
- Increasing Cycle Life: EFB and ALAB batteries offer longer cycle life, making them more durable and reliable.
- Enhancing Charge Acceptance: These batteries can accept charge more quickly, reducing charging time and improving efficiency.
- Improving Performance in Extreme Conditions: EFB and ALAB batteries offer better performance in extreme temperatures, making them suitable for a wider range of applications.
- Reducing Sulfation: Advanced designs and materials help reduce sulfation, extending battery life.
9.4 What Are the Future Trends in Lead-Acid Battery Technology?
Future trends in lead-acid battery technology include:
- Further Enhancements in Materials: Ongoing research into new materials to improve battery performance and lifespan.
- Integration with Smart Battery Management Systems (BMS): BMS to optimize charging and discharging, monitor battery health, and prevent damage.
- Development of More Sustainable Manufacturing Processes: Efforts to reduce the environmental impact of lead-acid battery production and recycling.
These advancements promise to make lead-acid batteries even more efficient, reliable, and environmentally friendly.
10. Lead-Acid Battery Purchase Guide
What should I consider when purchasing a new lead-acid battery? Selecting the right lead-acid battery is crucial for ensuring optimal performance and reliability.
10.1 What Are the Key Specifications to Consider?
- Voltage: Ensure the battery voltage matches the vehicle’s requirements (typically 12V for cars).
- Cold Cranking Amps (CCA): CCA indicates the battery’s ability to start the engine in cold temperatures.
- Reserve Capacity (RC): RC indicates how long the battery can power essential electrical components if the charging system fails.
- Battery Group Size: Ensure the battery group size matches the vehicle’s specifications.
- Ampere-Hour (Ah): Capacity is the amount of energy stored in the battery.
10.2 How Do I Choose the Right Battery for My Vehicle?
- Consult the Vehicle’s Owner’s Manual: The owner’s manual provides the recommended battery specifications.
- Consider the Climate: In colder climates, choose a battery with a higher CCA rating.
- Assess the Vehicle’s Electrical Load: If the vehicle has many electrical accessories, choose a battery with a higher RC rating.
- Compare Brands and Models: Research different brands and models to find a battery that meets your needs and budget.
10.3 What Are the Top Lead-Acid Battery Brands?
Some of the top lead-acid battery brands include:
- Interstate Batteries
- DieHard
- Optima Batteries
- ACDelco
- Exide
10.4 Where Can I Purchase High-Quality Lead-Acid Batteries?
High-quality lead-acid batteries can be purchased from:
- Automotive Supply Stores
- Online Retailers
- Battery Specialists
- CARDIAGTECH.NET
CARDIAGTECH.NET offers a wide selection of lead-acid batteries from top brands, ensuring you find the perfect battery for your needs. We also offer expert advice and support to help you make the right choice. Visit our website or contact us at +1 (641) 206-8880 for more information.
Equipping yourself with the right tools and knowledge ensures optimal vehicle performance and customer satisfaction. At CARDIAGTECH.NET, we understand the challenges you face. That’s why we offer a comprehensive range of high-quality automotive repair tools designed to enhance your efficiency, accuracy, and safety.
Don’t let outdated tools hold you back. Contact CARDIAGTECH.NET today at +1 (641) 206-8880 or visit our website CARDIAGTECH.NET to explore our extensive catalog. Located at 276 Reock St, City of Orange, NJ 07050, United States, we are ready to help you elevate your automotive repair capabilities and drive your business to new heights. Let us help you make every repair a success.
FAQ: Lead-Acid Batteries
1. What is the operating principle of a lead-acid battery?
The operating principle of a lead-acid battery involves reversible chemical reactions between lead dioxide (PbO2) and spongy lead (Pb) electrodes in a sulfuric acid (H2SO4) electrolyte, converting chemical energy into electrical energy during discharge and vice versa during charging.
2. How does a lead-acid battery produce electricity?
A lead-acid battery produces electricity through chemical reactions. During discharge, lead at the negative electrode reacts with sulfuric acid to form lead sulfate, releasing electrons. At the positive electrode, lead dioxide reacts with sulfuric acid and accepts these electrons, also forming lead sulfate.
3. What is the role of sulfuric acid in a lead-acid battery?
Sulfuric acid acts as the electrolyte in a lead-acid battery, facilitating the movement of ions between the electrodes. It participates in the chemical reactions that produce electrical energy during discharge and store energy during charging.
4. What happens to the electrolyte level in a flooded lead-acid battery over time?
Over time, the electrolyte level in a flooded lead-acid battery decreases due to water loss from evaporation and electrolysis during charging. It’s essential to periodically check and replenish the electrolyte level with distilled water to maintain proper battery function.
5. Can a lead-acid battery freeze in cold weather?
Yes, a lead-acid battery can freeze in cold weather if it is not fully charged. A discharged battery has a lower concentration of sulfuric acid and a higher concentration of water, making it more susceptible to freezing.
6. What is sulfation, and how does it affect lead-acid batteries?
Sulfation is the formation of lead sulfate crystals on the electrodes of a lead-acid battery. It occurs when a battery is left in a discharged state for an extended period, reducing its capacity and ability to accept a charge.
7. How often should I charge my lead-acid battery?
You should charge your lead-acid battery whenever it is significantly discharged, ideally before it drops below 50% state of charge. Regular charging helps prevent sulfation and extends the battery’s lifespan.
8. What is the difference between a starting battery and a deep-cycle battery?
A starting battery delivers a high burst of power for a short time to start an engine. A deep-cycle battery provides a steady amount of power over a longer period and is designed to be discharged and recharged repeatedly.
9. How can I properly dispose of a lead-acid battery?
Lead-acid batteries should be recycled properly to prevent environmental contamination. Take them to a recycling center, automotive supply store, or battery retailer that accepts used batteries.
10. What are the advantages and disadvantages of lead-acid batteries compared to lithium-ion batteries?
Advantages of lead-acid batteries include lower cost, higher surge current capability, and a more established recycling infrastructure. Disadvantages include lower energy density, heavier weight, and shorter cycle life compared to lithium-ion batteries.


